suPAR Biomarker – a leading innovation in prognostics
Improving patient outcomes and reduce healthcare costs by suPARcharging triage
The suPAR biomarker predicts critical illness across diseases, by measuring the level of activation in the immune response. suPARnostic® is a reliable prognostic tool that enables healthcare professionals to make quick and informed patient triaging decisions at emergency departments in hospitals.
Lead with confidence
suPARnostic® empowers healthcare professionals to lead patient triage with confidence by making better informed decisions, backed by reliable biological data across diseases.
Implementing the suPAR biomarker in clinical care delivers faster and safer patient discharges, reduces re-admissions, unnecessary admissions as well as hospital length of stay.
This frees critical resources, valuable time and saving hospital beds for those who need them.
Reduce healthcare costs
Up to £85 to £320 savings per admission depending on medical specialty and geography
Empower clinical staff
Get more information to make more confident decisions to potentially reduce uncertainty and stress
“It has become clear that suPAR is a very strong prognostic marker, exceeding the prognostic value of all other routinely measured biomarkers in our hospital.”
Prof. Ove Andersen,
MD, PhD, DmSc, Copenhagen University Hospital Hvidovre, Denmark
suPAR News Vol. 3, June 2020
suPAR is a protein in the blood that reflects immune activation. suPAR stands for soluble urokinase plasminogen activator receptor. All human beings have a baseline level of suPAR that is individually determined and increases with age.
High suPAR levels are associated with increased inflammation, disease progression, and risk of mortality in acute and chronic diseases, and in the general population.
Measuring suPAR levels can thus serve as a marker to determine who can be classified into low-risk category and their chances for survival upon hospital admission. suPAR can also be used for monitoring for prevention of disease progression and earlier intervention time point.
The suPAR level is elevated across diseases, and not solely associated with one specific disease. Therefore, suPAR is applicable as a prognostic marker and not as a diagnostic marker. This characteristic may be utilized for risk stratification in unselected patients.
The suPAR blood level is stable with no diurnal variation and no changes following fasting. It can be measured in blood, plasma, urine, cerebrospinal fluid, ascites fluid, and pleural fluid. The level increases and decreases with progression and improvement of a disease, respectively.
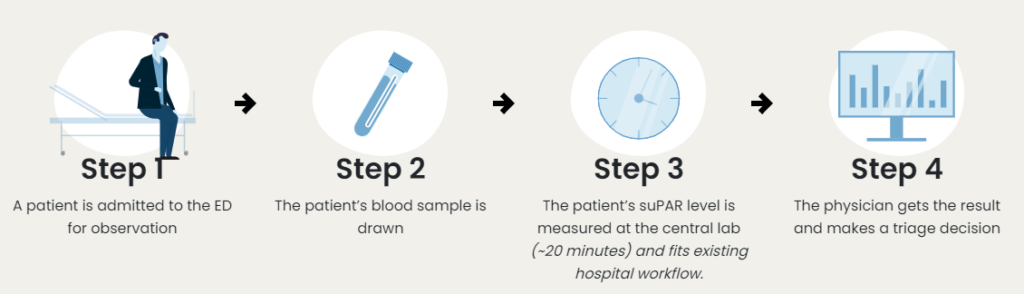
How suPARnostic® works in the Emergency Department

suPAR Biomarker – a leading innovation in prognostics
The suPAR biomarker predicts negative patient outcomes across diseases by measuring the immune system’s activation level. suPARnostic® is a reliable prognostic tool that enables healthcare professionals to make quick and informed patient triaging decisions at hospital emergency departments.
suPARnostic® empowers healthcare professionals to lead patient triage with confidence by making better informed decisions across diseases, backed by reliable biological data.
Implementing suPAR in clinical care saves critical resources and valuable time, and results in reduced healthcare costs, more efficient triage, and better patient outcomes.
At hospital emergency departments, suPAR delivers faster and safer patient discharges, reduces readmissions, avoids unnecessary admissions, and lowers hospital length of stay.
About suPAR and Virogates
ViroGates is an international medical technology company headquartered in Denmark, north of Copenhagen, in the heart of Medicon Valley. Virogates’ mission is to improve patient outcomes by empowering healthcare professionals to make better informed clinical decisions, leading to more efficient patient triage.
ViroGates was founded in 2001, based on an invention coming from Hvidovre Hospital, Denmark. Inventor and co-founder Jesper Eugen-Olsen discovered the utility of the biomarker suPAR in HIV. More than 750 peer reviewed publications have followed, substantiating suPAR as one of the most promising biomarker tools for various areas of healthcare.
ViroGates has developed a patented product line called suPARnostic® to make the suPAR test commercially available.
The suPAR biomarker predicts negative patient outcomes across diseases by measuring the immune system’s activation level. suPARnostic® is a reliable prognostic tool that enables healthcare professionals to make quick and informed patient triaging decisions at hospital emergency departments.
suPARnostic® empowers healthcare professionals to lead patient triage with confidence by making better informed decisions across diseases, backed by reliable biological data.
Implementing suPAR in clinical care saves critical resources and valuable time, and results in reduced healthcare costs, more efficient triage, and better patient outcomes.
At hospital emergency departments, suPAR delivers faster and safer patient discharges, reduces readmissions, avoids unnecessary admissions, and lowers hospital length of stay.
Seamlessly implemented by clinicians and laboratory technicians, suPARnostic products fit existing workflows in hospitals, are approved for all the major clinical chemistry analysers, and delivers results in less than 20 minutes.
Products
suPARnostic® is the only CE-IVD certified product range applied for clinical determination of suPAR (soluble uPAR) in human plasma and serum.
suPAR is a universal and broadly applicable prognostic biomarker of chronic inflammation and immune activation across diseases. suPAR can be used for risk stratification of acute medical patients.

suPAR is a universal and broadly applicable prognostic biomarker of chronic inflammation and immune activation across diseases and used for risk stratification of acute medical patients.
Important clinical benefits include:
- Prioritisation: Identifies patients who can be discharged after treatment.
- Safety: Identifies high-risk patients with unaffected vital signs.
- Cost savings: Shortens the average patient-length-of-stay and reduces the cost of healthcare.
suPARnostic® TurbiLatex products are approved for all major chemical analysers and fits existing hospital workflows. It is thus seamlessly implemented and delivers results in under 20 mins.
suPARnostic® TurbiLatex Instructions For Use

The suPARnostic® Quick Triage kit is used in the Point of Care situation for early warning and patient triaging.
The suPARnostic® Quick Triage, is an easy-to-use, quantitative test that will allow clinicians and nurses to obtain a fast patient prognosis for patient mortality upon hospital admission.
Test results can quickly be obtained on-site at the Emergency Department avoiding passing via the core laboratory.
The suPARnostic® Quick Triage test is used together with the aLF reader from Qiagen and delivers:
- Simple and easy-to-operate test
- Quick and fully quantitative suPAR results
- Early results for better triage of patients in an Emergency Department settings
suPARnostic® Quick Triage Instructions For Use

The CE/IVD marked suPARnostic® ELISA assay is a full quantifiable method.
The suPARnostic® ELISA assay is based on a simplified double monoclonal antibody sandwich ELISA* assay whereby samples and peroxidase-conjugated anti-suPAR are first mixed together and then incubated in anti-suPAR pre-coated microwells.
The recombinant suPAR standards of the kit are calibrated against healthy human blood donor samples.
suPAR concentrations are determined as ng/mL plasma.
Clinical Benefits of ELISA suPARnostic®:
- Triaging – Adds to clinical decision making, when selecting patients for admission.
- Predicting Patient Survival – suPARnostic® beats other biomarkers as well as most used clinical scores.